Ocean Biomedical, Inc. (NASDAQ: OCEA) Announces Patent Issued for PfGARP Malaria Antibodies Central to Company’s Malaria Treatment and Prevention Platforms
/EIN News/ -- Providence, RI, Aug. 27, 2024 (GLOBE NEWSWIRE) -- Ocean Biomedical (NASDAQ:OCEA) announced today that its Scientific Co-founder Dr. Jonathan Kurtis, MD, PhD, has been issued a key U.S. patent for his groundbreaking malaria therapeutic antibody discovery that targets PfGARP. Since publicizing news of his original research on PfGARP and its critical role in the malaria cycle in the Journal NATURE, Dr. Kurtis and his team have been working to deepen their understanding of how it naturally triggers the death of malaria parasites, and their control of that mechanism. Their expanded insights have already led to: 1.) a powerful vaccine candidate targeted for long term prevention of malaria infection; 2.) a therapeutic antibody candidate for short term malaria prevention; and 3.) a therapeutic small molecule drug candidate targeted to treat severe malaria, with potential to launch a whole new class of malaria medicines.
This patent is adding to Ocean Biomedical’s global patent portfolio of over 5 dozen patents for discoveries with potential to impact major unmet medical needs in infectious disease, oncology, and fibrosis, developed through grants totaling over $125M.
Kurtis’ novel approach causes parasite death at a key stage in the malarial cycle, triggering programmed cell death through apoptosis. This patent expands protection for Dr. Kurtis’ novel discoveries at a time when the most common strains of malaria are showing signs of growing resistance to current Artemisinin-based drugs.
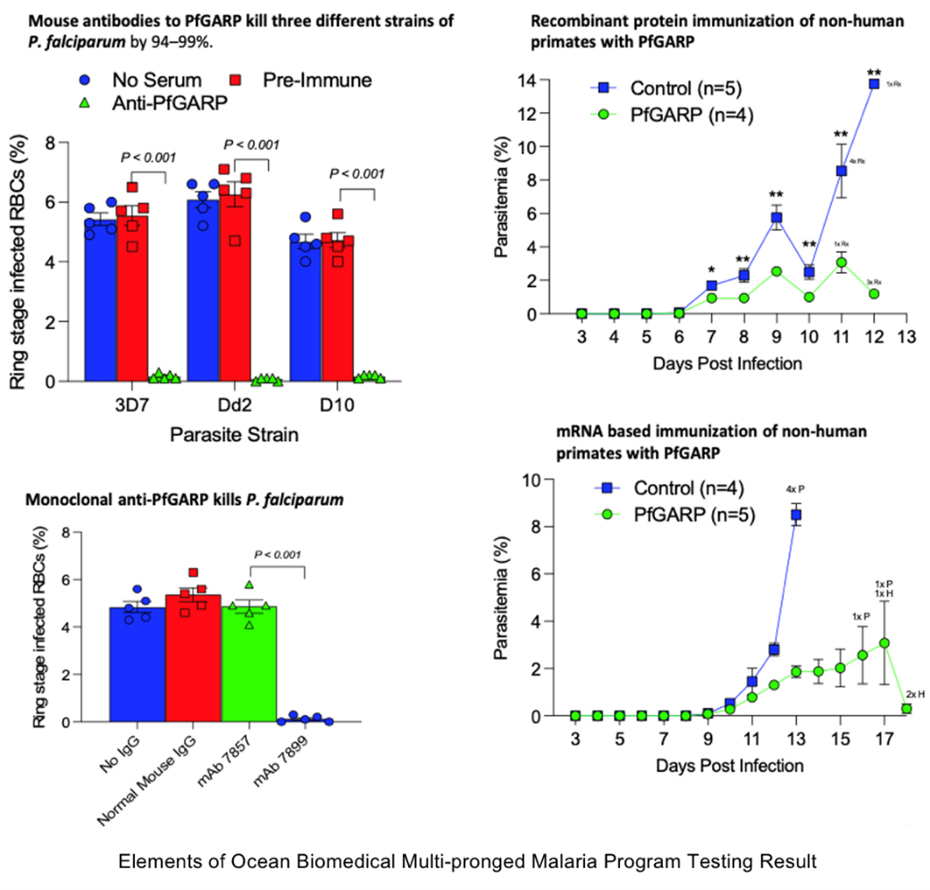
“Inducing parasite cell death via targeting PfGARP is a novel approach that has potential to result in a whole new class of anti-malarial interventions, including mRNA-based vaccines, small molecule drugs and our current monoclonal antibody,” said Dr. Kurtis. “Our monoclonal antibody and small molecule drug comes at a critical time because malaria parasites are developing resistance to current frontline therapeutics, and the currently approved vaccine offers only very limited protection.”

Dr. Jonathan Kurtis conducting research near Kisumu, Kenya, one of the world’s most malaria-infected regions
Addressing a Global Unmet Need
Malaria is the greatest single-agent killer of children on the planet, killing approximately 627,000 individuals in 2022. Artemisinin-based drug therapy remains the mainstay of treatment, but the spread of parasites resistant to this family of compounds threatens recent progress achieved by antimalarial campaigns and underscores the urgent need to identify new anti-malarial drugs.
Leadership Comments
“At each step in the process we are learning more about how this “kill switch” mechanism works to interrupt the malaria parasite’s lifecycle, and how we can exploit that on the prevention side and the treatment side,” commented Dr. Jake Kurtis, Scientific Co-founder of Ocean Biomedical, member of Ocean Biomedical’s board of directors and Chair of Pathology and Laboratory Medicine at the Warren Alpert Medical School Brown University.
“The progress we have been able to make thus far in advancing novel targets is a testament to Ocean Biomedical’s innovative model and deep partnership with premier research institutions. We are hopeful that Dr. Kurtis’ discoveries will lead to powerful new treatment options that can save hundreds of thousands of lives, and we are proud to be leading in this important work,” said Elizabeth Ng, Chief Executive Officer of Ocean Biomedical.
“The resurgence of Falciparum malaria worldwide is increasingly alarming and we are pleased to see Dr. Kurtis’ work receive this patent coverage for such an important discovery. We will work hard to accelerate the development of our novel vaccine candidate and this novel class of antimalarial antibodies to get a new treatment option for severe malaria available as soon as possible,” said Dr. Chirinjeev Kathuria, co-founder and Executive Chairman.
About Ocean Biomedical
Ocean Biomedical, Inc. is a Providence, Rhode Island-based biopharma company with an innovative business model that accelerates the development and commercialization of scientifically compelling assets from research universities and medical centers. Ocean Biomedical deploys the funding and expertise to move new therapeutic candidates efficiently from the laboratory to the clinic, to the world. Ocean Biomedical is currently developing five promising discoveries that have the potential to achieve life-changing outcomes in lung cancer, brain cancer, pulmonary fibrosis, and the prevention and treatment of malaria. The Ocean Biomedical team is working on solving some of the world’s toughest problems, for the people who need it most.
To learn more, visit www.oceanbiomedical.com
Forward-Looking Statements
The information included herein and in any oral statements made in connection herewith include “forward-looking statements” within the meaning of the “safe harbor” provisions of the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements may be identified by the use of words such as “estimate,” “plan,” “project,” “forecast,” “intend,” “will,” “expect,” “anticipate,” “believe,” “seek,” “target” or other similar expressions that predict or indicate future events or trends or that are not statements of historical matters, although not all forward-looking statements contain such identifying words. These forward-looking statements include, but are not limited to, statements regarding estimates and forecasts of financial and performance metrics and expectations. These statements are based on various assumptions, whether or not identified herein, and on the current expectations of the Company’s management and are not predictions of actual performance. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on by any investor as, a guarantee, an assurance, a prediction or a definitive statement of fact or probability. Actual events and circumstances are difficult or impossible to predict and will differ from assumptions.
The announced discoveries were based solely on laboratory and animal studies. Ocean Biomedical has not conducted any studies that show similar efficacy or safety in humans. There can be no assurances that this treatment will prove safe or effective in humans, and that any clinical benefits of this treatment is subject to clinical trials and ultimate approval of its use in patients by the FDA. Such approval, if granted, could be years away.
Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to risks and uncertainties. These forward-looking statements are not guarantees of future performance, conditions or results, and involve a number of known and unknown risks, uncertainties, assumptions and other important factors, many of which are outside the control of the Company that could cause actual results or outcomes to differ materially from those discussed in the forward-looking statements. Important factors, among others, that may affect actual results or outcomes include (i) the outcome of any legal proceedings that may be instituted against the Company; (ii) changes in the markets in which the Company competes, including with respect to its competitive landscape, technology evolution, or regulatory changes; (iii) changes in domestic and global general economic conditions; (iv) risk that the Company may not be able to execute its growth strategies; (v) risks related to the ongoing COVID-19 pandemic and response, including supply chain disruptions; (vi) risk that the Company may not be able to develop and maintain effective internal controls; (vii) the risk that the Company may fail to keep pace with rapid technological developments to provide new and innovative products and services or make substantial investments in unsuccessful new products and services; (viii) the ability to develop, license or acquire new therapeutics; (ix) the risk that the Company will need to raise additional capital to execute its business plan, which may not be available on acceptable terms or at all; (x) the risk that the Company experiences difficulties in managing its growth and expanding operations; (xi) the risk of product liability or regulatory lawsuits or proceedings relating to the Company’s business; (xii) the risk of cyber security or foreign exchange losses; (xiii) the risk that the Company is unable to secure or protect its intellectual property.
The foregoing list of factors is not exhaustive. You should carefully consider the foregoing factors and the other risks and uncertainties that are described in the Company’s Annual Report on Form 10-K for the year ended December 31, 2021 and its Quarterly Report on Form 10-Q for the quarter ended September 30, 2022, and which are described in the “Risk Factors” section of the Company’s definitive proxy statement filed by the Company on January 12, 2023, and other documents to be filed by the Company from time to time with the SEC and which are and will be available at www.sec.gov. These filings identify and address other important risks and uncertainties that could cause actual events and results to differ materially from those contained in the forward-looking statements. Forward-looking statements speak only as of the date they are made. Readers are cautioned not to put undue reliance on forward-looking statements. These forward-looking statements should not be relied upon as representing the Company’s assessments as of any date subsequent to the date of this filing. Accordingly, undue reliance should not be placed upon the forward-looking statements.
Ocean Biomedical Investor Relations info@oceanbiomedical.com
Ocean Biomedical Media Relations connect@oceanbiomedical.com
Attachments

Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release