DELTAREX-G FOR CAR-T CELL THERAPY INDUCED CYTOKINE RELEASE SYNDROME
AUTHORS PUBLISH ON THE USE OF DELTAREX-G IN CAR-T CELL THERAPY INDUCED CYTOKINE RELEASE SYNDROME (Front. Mol. Med. 4: doi: 10.3389/fmmed.2024.1461151)
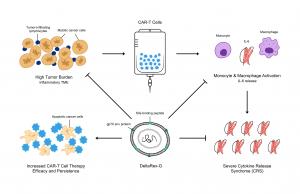
Haroun stated “This theory is supported by the inhibitory activity of DeltaRex-G in transduced CD4+ CD8+ cell cultures. DeltaRex-G may be used to treat CRS by inhibiting a certain proportion of the proliferative cytokine releasing immune cells, hence reducing production of IL-6, while retaining the efficacy of unaffected CAR-T cells (Figure). Clinical data from cancer patients treated with DeltaRex-G have shown an initial control of tumor growth with eventual tumor shrinkage and attainment of clinical remission after 8 months of DeltaRex-G therapy”.
Gordon further stated “Albeit DeltaRex-G has not yet been used to treat CRS, DeltaRex-G has not been reported to cause hematologic nor organ dysfunction in Phase 1 and Phase studies using DeltaRex-G in advanced sarcoma, pancreatic cancer and carcinoma of breast. Further no vector neutralizing antibodies have formed with prolonged DeltaRex-G therapy, indicating that DeltaRex-G is not immunogenic. Additionally, no delayed adverse events have been reported in long term (>15 years) cancer survivors with DeltaRex-G treatment. Nevertheless, a phase 1/2 clinical study is warranted to show the safety and inhibitory activity of DeltaRex-G in patients suffering from steroid resistant cytokine release syndrome following CAR-T cell therapy”.
For further information, please go to the following websites: www.avenifoundation.org or contact Dr. Gordon at egordon@avenifoundation.org or egordon@sarcomaoncology.com. To make a donation, please visit our website at www.avenifoundation.org and click on the “donate” button for credit card donations.
Erlinda Gordon
Aveni Foundation
+1 818-726-3278
egordon@avenifoundation.org
Distribution channels: Banking, Finance & Investment Industry, Healthcare & Pharmaceuticals Industry, Science, Technology
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release