Phenylketonuria Therapeutic at a Turning Point as Gene Therapy and Small Molecules Challenge Incumbents | CI Insights
Treatment paradigm for Phenylketonuria is rapidly evolving, fueled by breakthroughs in gene therapy, small molecule development & enzyme replacement strategies.
AUSTIN, TX, UNITED STATES, May 30, 2025 /EINPresswire.com/ -- The global Phenylketonuria (PKU) Treatment is undergoing a strategic transformation, driven by novel drug candidates and a more targeted understanding of unmet clinical needs. With a global prevalence of approximately one in 24,000 live births, PKU remains a rare but highly impactful genetic disorder caused by the body’s inability to break down phenylalanine (Phe), an amino acid present in many protein-rich foods. This defect, stemming from mutations in the PAH gene, can lead to severe neurological impairment if left untreated.
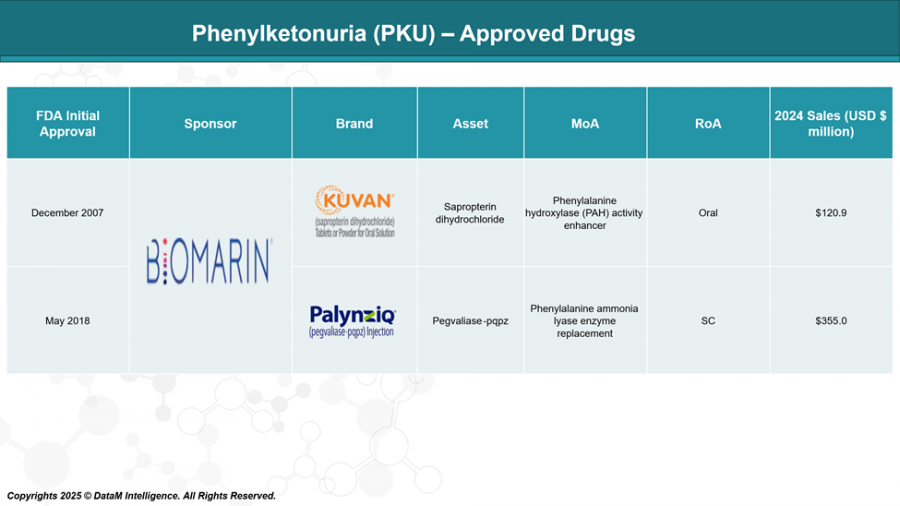
Historically, management of PKU has relied on strict dietary regulation and a small set of therapeutic options, primarily Kuvan (sapropterin dihydrochloride) and Palynziq (pegvaliase-pqpz), both developed by BioMarin Pharmaceutical. Kuvan, an oral BH4 cofactor supplement, serves as the frontline therapy for patients with mild to moderate PKU who are BH4-responsive. Palynziq, an injectable enzyme substitution therapy, is reserved for severe PKU patients and offers more robust Phe reduction, but it is associated with considerable immunogenic risk, including anaphylaxis, and requires frequent injections—factors that compromise long-term adherence.
Book Your Free Consultation Call: https://www.datamintelligence.com/strategic-insights/ci/phenylketonuria-pku
However, competitive pressure is mounting as several promising therapies near clinical or regulatory milestones, signaling a dramatic shift in how PKU may be treated in the coming years.
PTC Therapeutics is at the forefront with its lead candidate sepiapterin, a potent BH4 analog designed to offer broader efficacy across a larger population of BH4-responsive patients. Unlike Kuvan, sepiapterin is anticipated to demonstrate superior brain penetration and stronger Phe-lowering effects, potentially replacing Kuvan as the preferred oral therapy. Its once-daily oral route maintains the convenience of Kuvan while offering an enhanced therapeutic profile that could improve both biochemical outcomes and neurocognitive protection. Currently in pre-registration status, sepiapterin may soon become a serious contender in the first-line treatment space, particularly if it is priced competitively.
Meanwhile, Otsuka Pharmaceutical, in collaboration with Jnana Therapeutics, is developing Repinatrabit (JNT-517), a first-in-class small molecule that clears phenylalanine through the kidney. Unlike BH4-dependent therapies, Repinatrabit works independently of the PAH enzyme pathway, making it suitable for a broader range of patients, including those who are completely unresponsive to current therapies. As an oral, non-dietary treatment, it is especially appealing for patients struggling with adherence to protein-restricted diets. The differentiated mechanism of action and ease of administration position Repinatrabit as a potentially transformative option for patients who have been underserved by existing treatments.
On the innovation frontier, NGGT INC. is pushing forward with NGGT002, a gene therapy that aims to offer a one-time, potentially curative solution for all PKU patients, regardless of genotype or severity. NGGT002 uses a vector-based delivery system to restore PAH enzyme activity at the genetic level. If successful, this therapy could eliminate the lifelong burden of dietary restrictions, daily medication, and injection-based treatments. However, gene therapies come with their own set of commercial and regulatory hurdles, including the need to demonstrate long-term durability, manage manufacturing complexity, and secure reimbursement from payers. Nonetheless, NGGT002 represents a paradigm shift in PKU management with the potential to redefine the value proposition for rare disease treatment.
From a competitive intelligence perspective, the Target Opportunity Profile (TOP) for new entrants into the PKU market is now well defined. To successfully compete or displace existing therapies like Kuvan and Palynziq, emerging treatments must achieve several key benchmarks. These include significant and consistent reductions in blood phenylalanine levels—ideally above 60% across all patient types, including those unresponsive to BH4. Additionally, the new therapies must exhibit strong safety profiles, avoiding the immunogenic reactions and systemic side effects associated with biologics like Palynziq.
A novel or best-in-class mechanism of action will also be critical. Whether it's through renal clearance, gene correction, or synthetic biology, a new mode of action that bypasses current resistance and tolerance issues offers a compelling competitive edge. The route of administration will play a major role as well; oral or non-invasive therapies are strongly preferred due to their impact on patient adherence and quality of life. Ideally, dosing frequency should be as low as possible—once daily or even once weekly—with a fast onset of action, showing measurable clinical improvements within two to four weeks.
Moreover, the next wave of PKU treatments must demonstrate efficacy across all patient subtypes, including pediatric patients, adolescents, and individuals with severe, non-BH4 responsive forms of PKU. Beyond biochemical control, developers will need to show improvements in cognitive function, behavior, and quality of life—endpoints that are increasingly valued by regulators, payers, and caregivers alike.
BioMarin’s dominant market share, built on first-mover advantage and established prescriber relationships, now faces real threats. PTC’s sepiapterin is positioned to cannibalize Kuvan’s base with superior efficacy and brain penetrance. Otsuka’s Repinatrabit offers a novel mechanism that addresses key shortcomings of current therapies and could redefine the standard for maintenance care. NGGT’s gene therapy, while still in the early stages, presents a bold and disruptive vision for long-term disease management that could make traditional treatments obsolete in the next decade.
Download Free CI Sample: https://www.datamintelligence.com/strategic-insights/sample/phenylketonuria-pku
To stay ahead, pharmaceutical players are leveraging advanced CI tools and frameworks, including pipeline analysis, clinical trial tracking, social media listening, regulatory surveillance, and Key Opinion Leader (KOL) mapping. Real-time data from in-house platforms and external databases allow for precise market sizing, competitor benchmarking, and launch planning. These insights support smarter investments in pricing, reimbursement, and lifecycle strategies, ensuring that novel therapies align with both clinical value and commercial viability.
In a therapeutic area long dominated by dietary interventions and limited pharmacologic options, the PKU space is finally experiencing a wave of true innovation. The market is expanding, but so is the bar for entry. Only those therapies that combine efficacy, safety, convenience, and differentiated science will succeed in reshaping the future of PKU care.
Why Choose Our PKU Competitive Intelligence Report?
Our comprehensive CI report on Phenylketonuria offers an in-depth view of the current and future state of PKU therapeutics. We track every strategic development—from regulatory filings and trial results to KOL sentiment and payer activity—to give you a competitive edge in forecasting market shifts, spotting white space, and planning your next move.
Read More CI Report:
1. Rett Syndrome Research Report
2. Neurofibromatosis Type 1 Research Report
Sai Kiran
DataM Intelligence 4market Research LLP
+1 877-441-4866
email us here
Visit us on social media:
LinkedIn
X
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release