First Commercial Use of Jointechlabs’ Mini- Stem System in Italy, Greece, and Cyprus
Following CE-Mark approval, Jointechlabs' Mini-Stem Microfat system was successfully used in orthopedic and aesthetic procedures in Italy, Greece, and Cyprus.
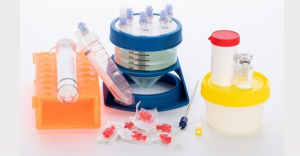
Mini-Stem System produces the purest regenerative microfat from a patient’s own body. Microfat procedures can be done in a local clinic, under local anesthesia, in as little as one hour. The provider injects the resultant microfat into the painful area in orthopedic situations. In aesthetic treatments, the provider uses microfat as a natural dermatologic filler.
● Orthopedic Use
The first Mini-Stem orthopedic patient in Italy underwent a procedure under the care of Dr. Marco Spoliti, a leading orthopedic and trauma surgeon. This marked a pivotal moment in the expansion of advanced medical treatments in the region.
● A Unique System
Mini-Stem System produces the purest microfat of any available device. And it is the only system in the world that goes beyond microfat and isolates and produces Stromal Vascular Fraction (SVF). SVF is an advanced regenerative substance which eager doctors and patients can now use.
Mini-Stem underwent rigorous testing and has also received CE-Mark approval, ensuring its safety and efficacy within EU healthcare systems. This achievement paves the way for broader adoption within the EU community. A sister device is well established in the US.
● Aesthetic Use
Microfat grafting offers benefits because it uses fat from the patient’s own body. Therefore the results tend to look more natural than those achieved with synthetic fillers. The transferred micronized fat cells contain the stromal cells that potentially will last years. By comparison, synthetic fillers may require frequent touch-ups.
Prominent physicians including Dr. Francesco Calvani, a maxillofacial surgeon, and renowned plastic surgeons Dr. Neophytos Demetriades and Dr. Angeles Karatzias performed aesthetic procedures in Italy, Greece, and Cyprus.
Nishit Pancholi, MD, COO of Jointechlabs, said “With the successful introduction of Mini-Stem System, Jointechlabs continues its mission of expanding access to regenerative medicine technologies worldwide. With our EU-based development and distribution partners, we are working with healthcare providers across the EU to train medical professionals and ensure the successful integration of Mini-Stem System for both orthopedic and aesthetic indications.”
● About Jointechlabs
Privately-held Jointechlabs is an emerging worldwide leader in point-of-care regenerative medicine. Jointechlabs enables healthcare practitioners and hospitals to provide safe, cost-effective, non-surgical regenerative medicine therapies. These therapies can be done at the point-of-care without change in infrastructure.
FDA-cleared MiniTC® for the US market is a stand-alone device for processing autologous fat into regenerative-cell-rich microfat for a variety of orthopedic, aesthetic, wound healing, and reconstructive surgery applications. Outside the US, CE-Mark-approved Mini-Stem System® prepares microfat and also extracts Stromal Vascular Fraction.
Pipeline products include stem-cell therapeutics such as 3D Bioprinted wound repair and injectable stem cell scaffolds.
More information: www.jointechlabs.com
Full story: https://jointechlabs.com/first-commercial-use-of-jointechlabs-mini-stem-system-in-italy-greece-and-cyprus/
Media Relations
Jointechlabs, Inc.
+1 800-217-0491
info@jointechlabs.com
Visit us on social media:
Facebook
X
LinkedIn
Instagram
YouTube
Other
Brilliant technology for microfat Mini-Stem Adipose Processing System
Distribution channels: Banking, Finance & Investment Industry, Companies, Healthcare & Pharmaceuticals Industry, Science, Sports, Fitness & Recreation
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release